As the COVID-19 pandemic emerges, scientists work day and night to produce effective therapies and vaccines against SARS-CoV-2, the virus responsible for the present epidemic. Numerous efforts focus on the coronavirus spike protein, which binds the angiotensin-converting enzyme 2 (ACE2) on human cells to permit viral ingress. Researchers currently provide information in ACS Central Science that have uncovered an active role for glycans — sugar molecules that can decorate proteins; this process suggests targets for therapies and vaccines.
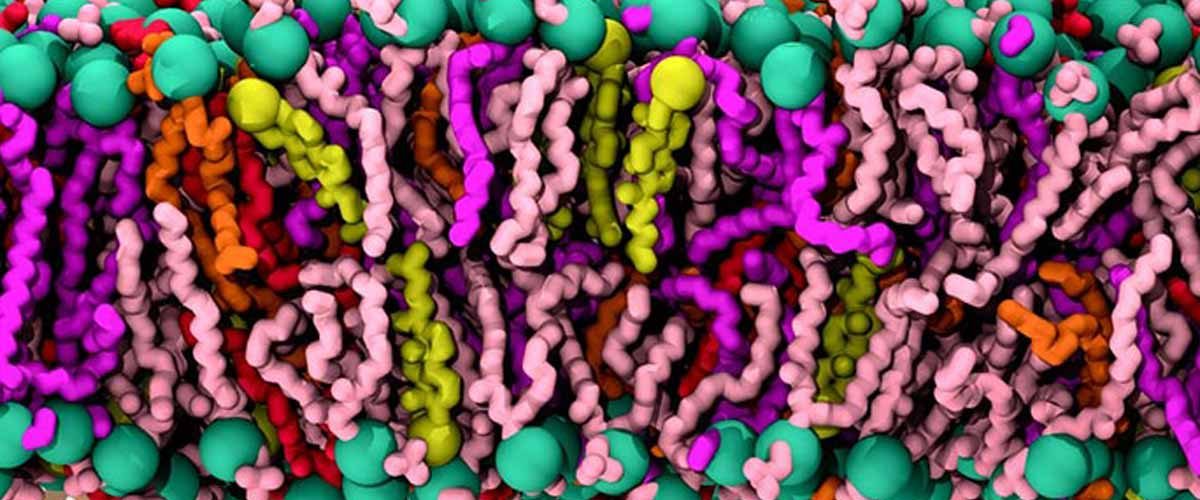
Before the SARS-CoV-2 spike protein can cooperate with ACE2 on a human cell, it alters shape to expose its receptor-binding domain (RBD), the part of the protein that interacts with ACE2. Like several viral proteins, the SARS-CoV-2 spike protein has a substantial coat of glycans on its surface. These glycans attached at definite sites facilitate to shield the viral proteins from the host immune system. Rommie Amaro and colleagues at the University of California San Diego, Maynooth University (Ireland), and the University of Texas at Austin inquired whether certain glycans in the SARS-CoV-2 spike protein might also be actively involved in the process leading to illness.
For determination, the researchers used glycomic and structural data to construct molecular dynamics simulations of the SARS-CoV-2 spike protein implanted in the viral membrane. The computerized models, which presented a comprehensive snapshot of every atom in the spike glycoprotein, revealed that N-glycans linked to the spike protein at definite sites (N165 and N234) aid stabilize the form change that exposes the RBD, which could help endorse infection. The simulations also identified regions of the spike protein that weren’t coated by glycans and could be defenseless to antibodies, particularly after the shape change. In laboratory experiments using biolayer interferometry, the group showed that mutating the spike protein stopped glycans at N165, and N234 reduced binding to ACE2. These findings lay the foundation for new policies to fight the pandemic risk, the researchers say. The authors acknowledge funding from the National Institutes of Health, the National Science Foundation, the Research Corporation for Science Advancement, UC San Diego Moores Cancer Center, the Irish Research Council, and the Visible Molecular Cell Consortium.











Current pandemic demands Spike protein-based vaccines and antiviral therapies
Informative