December 1, 2019 the day coronavirus disease (COVID-19) first hit the world then it has rapidly emerged as a pandemic viral infection and the disruption caused by COVID-19 pandemic is devastating. The COVID-19 pandemic has led to a dramatic loss of human life worldwide and presents an unprecedented challenge to public health. . Global healthcare systems have faced multiple challenges, ranging from the high number of patients to lack of approved therapeutic options. The World Health Organization, 2020 states that “there is no current evidence to recommend any specific COVID-19 treatment for patients with confirmed COVID-19”. Wu et al., 2020 reported that the world has been made strenuous efforts to explore effective therapeutic options against the virus.
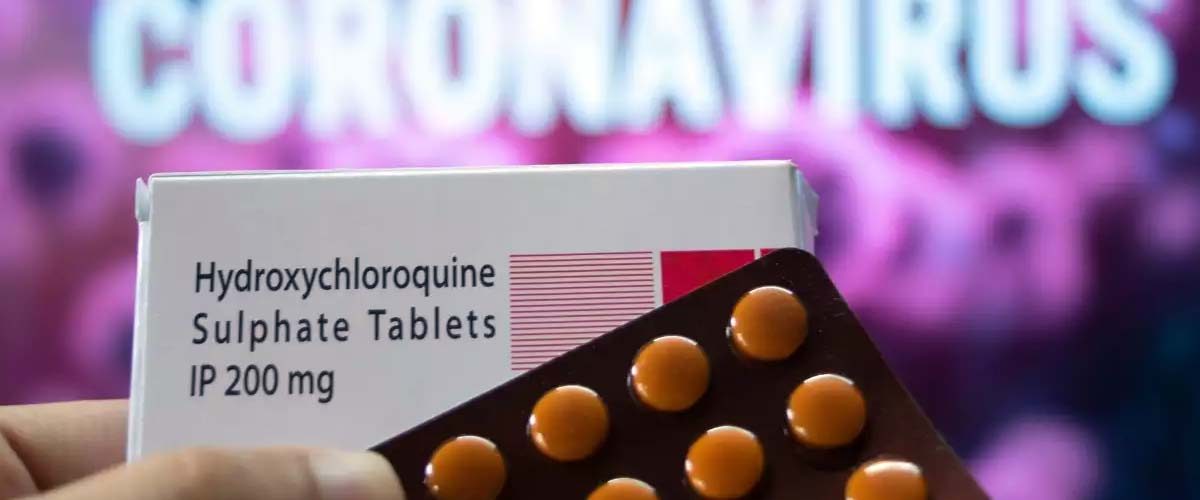
Liu et al., 2020 claimed that Hydroxychloroquine has antiviral effects in vitro, and in association with azithromycin was suggested to decrease severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a small non-randomised study. However, Geleris et al., 2020 stated that observational studies have suggested no beneficial effect of chloroquine or hydroxychloroquine in hospitalized patients with COVID-19.
The Saudi Arabian Ministry of Health (MOH) has launched an initiative in order to support the national healthcare system. Since 5 June 2020, 238 outpatient fever clinics have been established nationwide. The implemented protocol was based on hydroxychloroquine with zinc sulphate as the recommended treatment of choice.
A novel study was designed by Abdulrhman Mohana in collaboration with other researchers that is published in International Journal of Infectious Diseases in order to assess the safety outcomes and reported adverse events among COVID-19 patients attending outpatient fever clinics and subjected to the MOH-approved treatment protocol within 3–7 days in Saudi Arabia.
In a nutshell, the findings of this study showed that hydroxychloroquine use for COVID-19 patients with mild to moderate symptoms in an outpatient setting with the recommended protocol and inclusion/exclusion criteria is safe, is highly tolerable and has minimal side effects.










Add comment